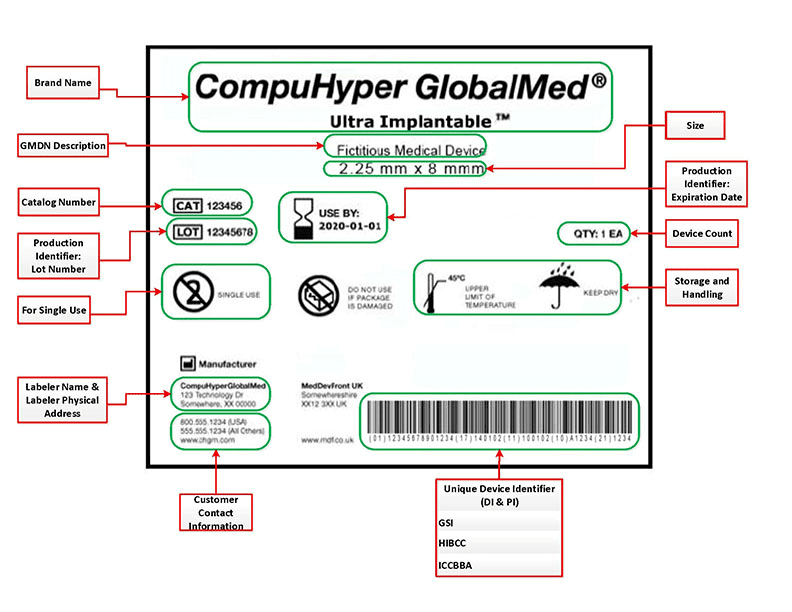
Medical Device Labels for FDA Unique Device Identifiers (UDI)
Electronic Imaging Materials (EIM) builds labeling that keeps pace with regulated manufacturing. Your devices need identification that scans cleanly, survives sterilization, and passes audits. Our team engineers medical device labels that do exactly that, from pilot to full production.


Marsha R. | PhoenixSongs Biologicals, Inc.
“You have always taken care of our labeling needs and we appreciate that.“

FDA Compliant Labels, Built by EIM
Confidence starts with construction. EIM specifies films, adhesives, and print systems that support UDI data structure and

UDI Codes That Survive Sterilization and Stay Readable
Traceability depends on contrast, quiet zones, and durable imaging. EIM designs UDI labels for medical devices that deliver crisp 1D and 2D codes, verified against your scanners and lighting. When your workflow includes steam, EtO, or aggressive disinfectants, we pair resin ribbons and protective topcoats to keep codes intact. Your medical device labels remain scannable when it matters most.

Labels Built for Clinical Workflows and Tough Surfaces
Surfaces and environments vary, but performance should not. EIM’s medical device labeling solutions use proven films such as polyester and polyimide, matched with high-tack adhesives for stainless steel, coated metals, glass, and challenging plastics. Thermal transfer imaging preserves microtext and UDI symbols. Where chemistry and abrasion are tough, laminates extend label life so your medical device labels stay in spec.
Get UDI Labeling Dialed In
Request free UDI label samples matched to your surfaces, sterilization steps, and scanners. An EIM specialist will recommend materials, adhesives, and print specs so your labels meet requirements and perform consistently.
Case Studies: Proof From the Field
Electronic Imaging Materials (EIM) builds labeling that keeps pace with regulated manufacturing. Your devices need identification that scans cleanly, survives sterilization, and passes audits. Our team engineers medical device labels that do exactly that, from pilot to full production.
Saving Arteries
One Label at a TimeA vascular research team needed labels that endured freezing, thawing, and constant handling. EIM delivered a cryo-ready construction with high-contrast print that reduced relabeling and helped protect sample integrity. Results included faster scans and fewer interruptions in critical workflows.
Aiding AIDS Research
Legibility after storage and processing was essential for a program managing sensitive clinical samples. EIM specified a resin ribbon and durable film that maintained barcode grades through the lab’s cleaning protocol. Technicians reported consistent reads and smoother handoffs across departments.
What our customers are saying
Real feedback, real results. Teams trust Electronic Imaging Materials for responsive service, fast turnarounds, and labels that perform under pressure from bench to bedside.

Cory M. | Schneider Regional Medical Center
“Everyone that I spoke to or communicated with was professional and helped with any and everything that I needed.”

Allison K. | CarolinaEast Health System
“I had ordered our yearly labels later than usual this year. However, EIM was able to deliver our new labels before the beginning of the new year.”

Marco F. | Strados Labs
“They were able to provide us with a high-performance customized solution for our medical device in a very short amount of time during the busy season.”

Ethan H. | Johnson & Johnson
“It was packaged well. The product was securely packed in durable packaging. I was impressed.”

Tom P. | Dartmouth
“Electronic Imaging Materials, Inc. is our first choice for our labeling and scanning requirements. Their customer service is top shelf and is always ready to supply our needs.”
Choose the Right UDI Label With EIM
Make selection fast and repeatable with this quick checklist. EIM specialists can validate each item on your actual parts and process before you approve a construction.
- Surface compatibility: EIM tests adhesion and edge hold on your exact substrate and finish. Stainless steel, coated metals, glass, and challenging plastics are all supported.
- Environment: Together we map heat, moisture, chemicals, and sterilization in process order, including autoclave, EtO, and common disinfectants.
- Lifespan: We help define single-use or reusable cycles and cleaning frequency, then set performance targets for handling, storage, and transport.
- Print method: EIM recommends resin ribbons where harsh processes require lasting barcodes. We verify barcode grades with your scanners after each process step.