CryoLabel® Cryogenic Labels
Electronic Imaging Materials, Inc. manufactures customized cryogenic labels used in the fields of virology, genetics, DNA sequencing, drug discovery, forensics and related laboratory sciences. Our specially engineered CryoLabel® line handles cryogenic temperatures and adheres to a variety of vials, test tubes and plates. Select freezer labels solutions ready to ship! See how a fertility treatment center in Canada uses our labels.

Our cryogenic labels:

Adhere to already frozen surfaces

Stick well around the curve of tight diameter vials

Allow the contents of a vial to remain visible
(available in very small sizes)

Resist moisture through repeated freeze and thaw cycles
Fit small side walls on microwell, micro assay and Microtiter® plates

Can be printed in color, custom thermal transfer printing
Are available in blanks (Blank CryoLabel®), which you can print onsite
Need an even more glove-friendly cryo solution? Check out our Easy Peel CryoLabel® Frost! >
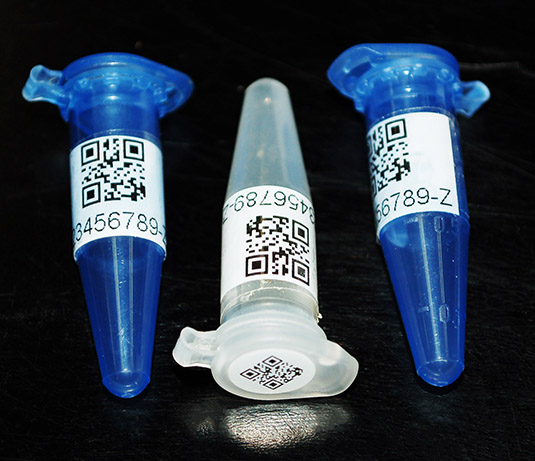
Cap and Wrap® CryoLabel® for Cold Temperature Tube Storage
Developed specifically for automated retrieval systems, our Cap and Wrap® Label Sets are made with our thermal transfer printable CryoLabel® they handle liquid nitrogen temperatures (-196⁰C) as well as water baths, solvents and chemicals such as IPA and DMSO.
This two-label set consists of a small round label for the tube cap (perfect for a 2D barcode) and a rectangular tube label. Designed so you can easily see both the contents of your tube and a barcode, the tube label has an opaque print block and a long tail of clear material to wrap around your tube and back over your printed information for protection.
Review our label product specifications and make sure these ultra-durable labels meet your requirements. Still not sure if these labels are right for your application? We’ll send you free label samples to test >
Label Materials
-
667 White CryoLabel® Plastic
Cold-temp film can be preprinted with color bars & logos. Works well on small diameter polypropylene tubes. Thermal transfer printable. -
685 Clear CryoLabel® Plastic
Flexible film with high-tack, glove-friendly adhesive for freezer labels in cryogenic conditions. Print block lets you add barcodes & HRI. Wraps easily around small tubes & back over the printed area for extra protection, while also letting you see tube contents. Thermal transfer printable. -
686 White CryoLabel® Frost Polyester
Thin, flexible, and tough film for wrapping around frozen test tubes, in freezers, LN2, autoclave, incubation, and boiling water processes. Water, scratch, chemical & solvent resistant. Thermal transfer & color printable. NOTE: Requires use of external rewind to print with a desktop printer. Available as a Cap and Wrap® Label Set. -
687 Clear CryoLabel® Frost Polyester
Clear film to wrap around test tubes & over printed data; lets you see tube contents. High quality, full-color printing. Water, scratch, chemical & solvent resistant. For frozen tubes, in freezers, LN2, autoclave, incubation & boiling water processes. Thermal transfer printable. NOTE: Requires use of external rewind to print with a desktop printer. Available as a Cap and Wrap® -
562 White CryoLabel® Plastic
Strong, yet flexible matte poly-blend suitable for frozen tubes. Glove friendly. Thermal Transfer & Color Printable. -
267 White CryoLabel® Plastic
Direct thermal film with a high tack adhesive. Useful for HIPAA. -
568 White CryoLabel® Plastic
Designed for cryogenic applications such as test tubes and ampules. This biaxially oriented polypropylene has an aggressive cryogenic rubber-based adhesive. -
668 Freez-R-MarkTM
This plastic label has a glossy finish and a strong adhesive that can bond to flexible packaging, as well as varnished or waxed corrugated materials, even in freezing temperatures. It is perfect for applying labels at room temperature and then subjecting them to -40ºF blast freezing. The plastic adheres to surfaces that are frosty or moist and works well on textured surfaces.
Microtiter® is a registered trademark of Thermo Labsystems, Helsinki, Finland.
CryoLabel® is the registered trademark of Electronic Imaging Materials, Inc., Keene, NH 03431 USA
What our customers are saying

Kuda M. | Zvitambo
“Been a customer for years and happy with product and service and therefore no real reason to switch or use another product or service provider. Also good to have consistency in product quality..cryolabels and ribbon.“

Adam B. | Arnold Lumber
“EIM has always been a fantastic company to work with; I recommend your products and services to all the other Epicor BisTrack and WMS users we interact with all over the United States and Canada.“

Phil B. | OriGen Biomedical
“Like the saying goes, “If it ain’t broke, don’t fix it”.“

Marina K. | SPT Labtech
“I am working with Jeremiah Rutherford. Great service. Responds right away. Any questions asked answered right away. Very professional. Outstanding service!!!“
We’re Ready To Help!
Talk With A Label Expert
