Medical Device Labels for FDA Unique Device Identifiers (UDI)
Durable Industrial Labels for Class I, II & III Medical Devices
We specialize in meeting government regulations & aggressive material standards for industrial strength labels. Our medical device labels meet the FDA’s durability requirements as prescribed by UL/IEC 60601-1, 3rd edition.
Product Highlights
Permanent solutions for device life cycle traceability

Maintains bond through sterilization cycles
Options for single-use & multi-use devices

Meets FDA standards
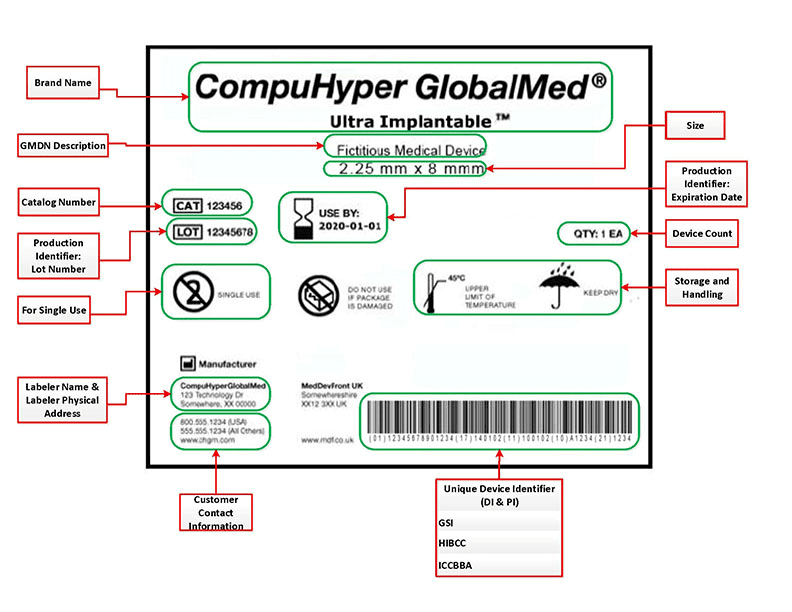
FDA Unique Device Identifier labels must contain the following requisite information:
> Name & address of manufacturer, packer or distributor
> Unique device identifier (UDI) code
> Expiration date
> Control number (if device is subject to 21 CFR Part 820.65)
> Storage & handling instructions
> Indications of use*
Our medical device labels are available in several material options.
If you are interested in evaluating samples of materials, contact us to be sent free labels to test in your manufacturing facility.
Review our label product specifications and make sure these ultra-durable labels meet your requirements. Still not sure if these labels are right for your application? We’ll send you free label samples to test >
Label Materials
-
628 Gloss White Polyester
Special adhesive technology, designed for textured, low surface energy plastics. -
640 Gloss White Polyester
Suited for metal surfaces & temperature extremes. Also available in silver (622). -
690 Opaque White Polyester
Offers good durability; excellent heat, tear & chemical resistance. Also available in silver (322).
What our customers are saying

Adam C. | MediView
“Marissa Salisbury went above and beyond with our initial calls and then subsequent emails/calls. She has been a godsend and helped us make the best decision for our organization including pointing us in the right direction with UDI compliance.“

Marco F. | Strados Labs
“They were able to provide us with a high-performance customized solution for our medical device in a very short amount of time during the busy season.“

Riley P. | Adaptas
“I appreciate the quick turn around on the order EIM was able to offer and their customer service. I had questions as a new customer, but the team was able to respond quickly to my inquiries. great communication.“

Michaela B. | NAMSA
“Very quick and professional customer service! I would absolutely order again. It is wonderful to be able to order labels on a fast and ad hoc/a la carte basis VS requiring the labels to be included in another service package, etc.“
We’re Ready To Help!
Talk With A Label Expert
